
Chemistry, 06.05.2020 20:04 MikeWrice4494
Mercury(II) oxide (HgO) decomposes to form mercury (Hg) and oxygen (O2). The balanced chemical equation is shown below.2HgO Right arrow. 2Hg + O2The molar mass of HgO is 216.59 g/mol. The molar mass of O2 is 32.00 g/mol. How many moles of HgO are needed to produce 250.0 g of O2?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1


Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Mercury(II) oxide (HgO) decomposes to form mercury (Hg) and oxygen (O2). The balanced chemical equat...
Questions in other subjects:

Mathematics, 25.06.2019 11:00



Mathematics, 25.06.2019 11:00


Mathematics, 25.06.2019 11:00

English, 25.06.2019 11:00


Business, 25.06.2019 11:00

Social Studies, 25.06.2019 11:00

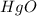 are needed to produce 250.0 g of
are needed to produce 250.0 g of 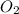
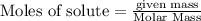
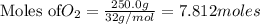
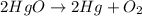
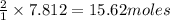 of
of 

