Chemistry, 25.06.2019 11:00 MyChannelBruh6896
Asample of atmospheric gas collected at an industrial site is stored in a 250 ml amber glass bottle that has a pressure of 1.02 atm and a temperature of 20.3°c. it was placed in an ice chest whose internal temperature is -2.0°c. what will be the new pressure of the gas sample once the gas temperature in the jar equilibrates to that of the ice chest?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 19:50, mikaylaaaaa
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
Asample of atmospheric gas collected at an industrial site is stored in a 250 ml amber glass bottle...
Questions in other subjects:

Mathematics, 12.01.2021 20:00

Mathematics, 12.01.2021 20:00




English, 12.01.2021 20:00

Chemistry, 12.01.2021 20:00



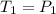
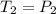
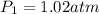 ,
, 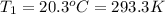
 ,
,