
Chemistry, 05.05.2020 16:31 donttrip10
150 mL of 0.15 M Na2SO4 is mixed with an equal volume of 0.050 M Agno3. Select all the options that correctly show the steps used to determine whether or not a precipitate will form, if Ksp for Ag2SO4

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
150 mL of 0.15 M Na2SO4 is mixed with an equal volume of 0.050 M Agno3. Select all the options that...
Questions in other subjects:

Mathematics, 08.11.2019 19:31





Chemistry, 08.11.2019 19:31




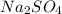 is mixed with an equal volume of 0.050 M
is mixed with an equal volume of 0.050 M 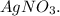
![[Ag^+] = \frac{0.05*150}{(150+150)}](/tpl/images/0640/2386/4c08d.png)
![[Ag^+] = \frac{7.5}{(300.0)}](/tpl/images/0640/2386/9fd3c.png)
![[Ag^+] = 0.025 \ M](/tpl/images/0640/2386/b8a04.png)
![[SO_4^{2-}] = \frac{0.15*150.0}{150.0+150.0}](/tpl/images/0640/2386/0ba50.png)
![[SO_4^{2-}] = \frac{22.5}{300.0}](/tpl/images/0640/2386/14cdb.png)
![[SO_4^{2-}] = 0.075 \ M](/tpl/images/0640/2386/c5d6b.png)
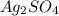 ;
; ![[Ag^+]^2 [SO_4^{2-}]](/tpl/images/0640/2386/8b0ad.png)
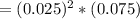
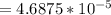
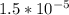 ;
; 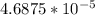 is < Ksp of
is < Ksp of 

