Chemistry, 06.05.2020 03:28 alwaysneedhelp84
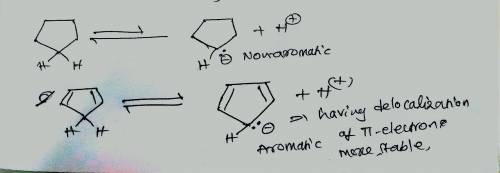
The pKapKa of cyclopentane is > 60, which is about what is expected for a hydrogen that is bonded to an sp3sp3 carbon. Explain why cyclopentadiene is a much stronger acid (pKapKa of 15), even though it too involves the loss of a proton from an sp3sp3 carbon. Match the words in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting your answer.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2
You know the right answer?
The pKapKa of cyclopentane is > 60, which is about what is expected for a hydrogen that is bonded...
Questions in other subjects:

Mathematics, 14.01.2021 23:00

Mathematics, 14.01.2021 23:00



English, 14.01.2021 23:00

Advanced Placement (AP), 14.01.2021 23:10


Mathematics, 14.01.2021 23:10