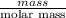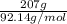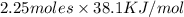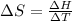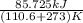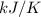Chemistry, 22.04.2020 23:45 phillipselijah2
The heat of vaporization Δ1, of toluene (C6H5CH3) is 38.1 kJ/mol. Calculate the change in entropy AS when 207. g of toluene boils at 1 10.6 °C. Be sure your answer contains a unit symbol. Round your answer to 3 significant digits.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

You know the right answer?
The heat of vaporization Δ1, of toluene (C6H5CH3) is 38.1 kJ/mol. Calculate the change in entropy AS...
Questions in other subjects:

English, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


English, 20.09.2020 01:01

English, 20.09.2020 01:01

History, 20.09.2020 01:01

Chemistry, 20.09.2020 01:01

Geography, 20.09.2020 01:01


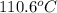 is 223
is 223 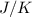 .
.