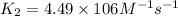Chemistry, 21.04.2020 18:50 xxaurorabluexx
For the second-order reaction NO( g) + O 3( g) → NO 2( g) + O 2( g), the rate constant has been measured to be 1.08 × 10 7 M –1 s –1 at 298 K and the activation energy has been measured to be 11.4 kJ/mol over the temperature range 195 K to 304 K. What is the rate constant at 207 K? ( R = 8.3145 J K –1 mol –1)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
For the second-order reaction NO( g) + O 3( g) → NO 2( g) + O 2( g), the rate constant has been meas...
Questions in other subjects:


History, 16.10.2019 16:30



English, 16.10.2019 16:30


History, 16.10.2019 16:30

Mathematics, 16.10.2019 16:30

Mathematics, 16.10.2019 16:30

History, 16.10.2019 16:30

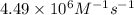
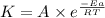
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0615/2426/6d953.png)
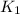 = rate constant at
= rate constant at 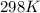 =
= 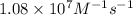
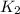 = rate constant at
= rate constant at 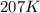 = ?
= ?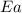 = activation energy for the reaction =
= activation energy for the reaction = 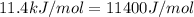
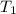 = initial temperature = 298 K
= initial temperature = 298 K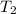 = final temperature = 207 K
= final temperature = 207 K![\log (\frac{K_2}{1.08\times 10^7M^{-1}s^{-1}})=\frac{11400J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{298K}-\frac{1}{207K}]](/tpl/images/0615/2426/e96a1.png)