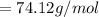Chemistry, 21.04.2020 18:07 roseemariehunter12
The following information is given for ether, C2H5OC2H5, at 1atm: boiling point = 34.6 °C Hvap(34.6 °C) = 26.5 kJ/mol specific heat liquid = 2.32 J/g°C /At a pressure of 1 atm, what is H in kJ for the process of condensing a 22.5 g sample of gaseous ether at its normal boiling point of 34.6 °C.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2
You know the right answer?
The following information is given for ether, C2H5OC2H5, at 1atm: boiling point = 34.6 °C Hvap(34.6...
Questions in other subjects:






Mathematics, 24.08.2019 02:10



Biology, 24.08.2019 02:10

Mathematics, 24.08.2019 02:10

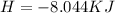
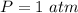

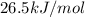

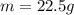
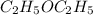 is given as
is given as