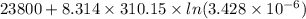The standard free-energy change for this reaction in the direction written is 23.8 kJ/mol. The concentrationsof the three intermediates in the hepatocyte of a mammal are: fructose 1,6-bisphosphate,1.4X10-5 M; glyceraldehyde 3-phosphate, 3X10-6 M; and dihydroxyacetone phosphate, 1.6X10-5 M. At body temperature (37C), what is the actual free-energy change for the reaction

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1


Chemistry, 23.06.2019 01:00, Johnson926
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
The standard free-energy change for this reaction in the direction written is 23.8 kJ/mol. The conce...
Questions in other subjects:










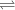 Glyceraldehyde 3-phosphate + DHAP
Glyceraldehyde 3-phosphate + DHAP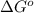 is 23.8 kJ/mol.
is 23.8 kJ/mol. 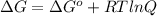
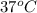
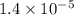 M
M
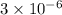 M
M
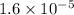 M
M ![\frac{[DHAP][\text{glyceraldehyde 3-phosphate}]}{[/text{Fructose 1,6-bisphosphate}]}](/tpl/images/0603/3958/6ad8b.png)
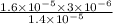
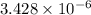
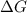 as follows.
as follows.