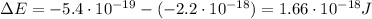Chemistry, 13.04.2020 11:27 jrfranckowiak
1) Green laser pointers are becoming popular for presentations. The green light is monochromatic
and has a wavelength of 532 nm.
a) What is the frequency of this light?
b) What is the energy in Joules of one photon of this light?
c) What is the energy in Joules of one mole of photons of this light?
d) What is the orbital energy in Joules of an electron in the ground state (n=1) in a hydrogen atom
according to the Bohr model?
e) What is the orbital energy in Joules of an electron in the n=2 state in a hydrogen atom according to
the Bohr model?


Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 15:30, alaf05160
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks. energy was destroyed inside the blocks. energy was absorbed into the blocks from outside the system. energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
1) Green laser pointers are becoming popular for presentations. The green light is monochromatic
Questions in other subjects:


Chemistry, 15.04.2021 19:40

Mathematics, 15.04.2021 19:40

Mathematics, 15.04.2021 19:40

Social Studies, 15.04.2021 19:40





Mathematics, 15.04.2021 19:40

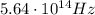
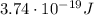
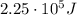
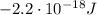
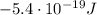
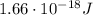
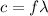
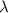 is the wavelength
is the wavelength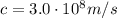 is the speed of light
is the speed of light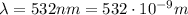 is the wavelength of the green light emitted by the laser
is the wavelength of the green light emitted by the laser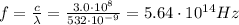
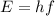
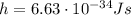 is the Planck's constant
is the Planck's constant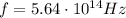 is the frequency of the photon
is the frequency of the photon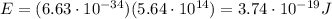
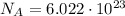
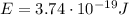
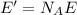
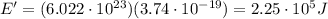
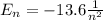 [eV]
[eV]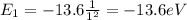
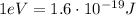
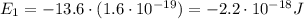
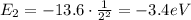
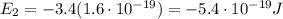
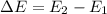
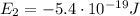 is the energy of orbital n=2
is the energy of orbital n=2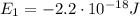 is the energy of orbital n=1
is the energy of orbital n=1