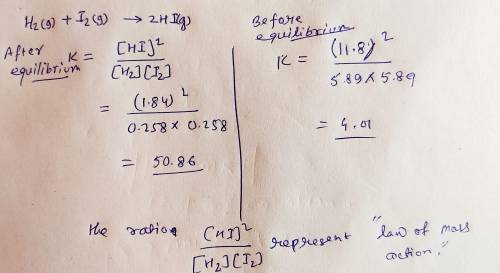
Onsider the chemical system: H2(g)+I2(g)>2HI(g)
5.89 mol H2, 5.89 mol I2, and 11.8 mo...

Chemistry, 07.04.2020 04:59 decoreyjpaipxv
Onsider the chemical system: H2(g)+I2(g)>2HI(g)
5.89 mol H2, 5.89 mol I2, and 11.8 mol HI are placed in a closed 10.00 L container maintained at 448°C. At equilibrium, the concentrations of H2, I2, and HI are 0.258 , 0.258 , and 1.84 M.
A. The ratio
[HI]2 / [H2][I2]
represents: ?
choose one or more:
1. the law of mass action.
2. the mass action expression.
3. the equilibrium constant expression.
4. the equilibrium constant.
5. none of these.
B. The value, 4.01 , represents: ?
choose one or more:
1. the law of mass action.
2. the mass action expression.
3. the equilibrium constant expression.
4. the equilibrium constant.
5. none of these.
C. The value, 50.9 , represents: ?
choose one or more:
1. the law of mass action.
2. the mass action expression.
3. the equilibrium constant expression.
4. the equilibrium constant.
5. none of these.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 15.04.2020 22:48

Mathematics, 15.04.2020 22:48





English, 15.04.2020 22:49



![\frac{[HI]^{2} }{[H_{2}[I_{2} ] }](/tpl/images/0586/0737/3585a.png) represent
represent