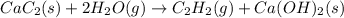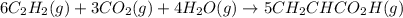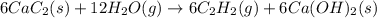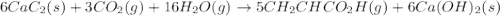Chemistry, 31.03.2020 00:21 isabelperez063
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide and water react to form acetylene and calcium hydroxide: (s)(g)(g)(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: (g)(g)(g)(g) Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1


Chemistry, 23.06.2019 00:00, familyk0jj3
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of...
Questions in other subjects:


Mathematics, 13.11.2020 03:20

History, 13.11.2020 03:20

Mathematics, 13.11.2020 03:20


Mathematics, 13.11.2020 03:20


English, 13.11.2020 03:20

English, 13.11.2020 03:20