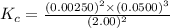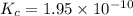Chemistry, 30.03.2020 23:54 dannaasc5475
1 Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) + 3O2(g) When this reaction was run at room temperature, the following equilbrium concentrations were measured: [O2] = 0.0500 M; [KCl] = 0.00250 M; [KClO3] = 2.00 M What is the equilibrium constant for this reaction?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, milesjreece3939
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 04:10, tishfaco5000
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

You know the right answer?
1 Potassium chlorate decomposes to product potassium chloride and oxygen gas. 2KClO3(s) ⇔ 2KCl(s) +...
Questions in other subjects:

Mathematics, 19.05.2021 23:40

Biology, 19.05.2021 23:40

Mathematics, 19.05.2021 23:40






Mathematics, 19.05.2021 23:40

Mathematics, 19.05.2021 23:40

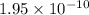
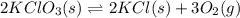
![K_c=\frac{[KCl]^2\times [O_2]^3}{[KClO_3]^2}](/tpl/images/0571/9600/641b5.png)