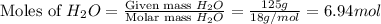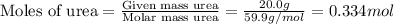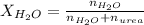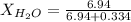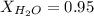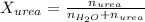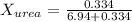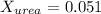Chemistry, 26.03.2020 19:59 nerikzagallegos
. You prepare a solution by adding 20.0 g Urea [(NH2)2CO] to 125 g water at 25.0oC & vapor pressure 23.76 torr. The solution vapor pressure is 22.67 torr. Calculate the molecular weight of urea. XH2O = moles H2O = Xsolute = moles urea = molar mass = calculations:

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:20, ashiteru123
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2


Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
. You prepare a solution by adding 20.0 g Urea [(NH2)2CO] to 125 g water at 25.0oC & vapor press...
Questions in other subjects:

Biology, 29.11.2019 13:31

Mathematics, 29.11.2019 13:31

Mathematics, 29.11.2019 13:31







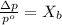
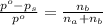
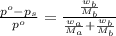
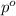 = vapor pressure of the pure solvent water = 23.76 torr
= vapor pressure of the pure solvent water = 23.76 torr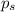 = vapor pressure of the solution = 22.67 torr
= vapor pressure of the solution = 22.67 torr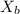 = mole fraction of solute (urea)
= mole fraction of solute (urea)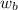 = mass of urea = 20.0 g
= mass of urea = 20.0 g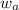 = mass of water = 125 g
= mass of water = 125 g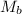 = molar mass of urea = ?
= molar mass of urea = ?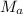 = molar mass of water = 18 g/mol
= molar mass of water = 18 g/mol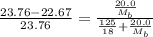
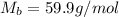
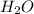 and urea.
and urea.