
The dimerization of butadiene 2C4H61 g2h C8H121 g2 was studied at 500. K, and the following data were obtained: Time (s) [C4H6] (mol/L) 195 1.6 3 1022 604 1.5 3 1022 1246 1.3 3 1022 2180 1.1 3 1022 6210 0.68 3 1022 Assuming that Rate 52 D3C4H64 Dt determine the form of the rate law, the integrated rate law, and the value of the rate constant for this reaction.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
You know the right answer?
The dimerization of butadiene 2C4H61 g2h C8H121 g2 was studied at 500. K, and the following data wer...
Questions in other subjects:


English, 18.08.2020 22:01


English, 18.08.2020 22:01






![k[C_4H_6]^2](/tpl/images/0561/6934/d2fdb.png)
![\frac{1}{[C_4H_6]}=\frac{1}{[C_4H_6]_0}+kt](/tpl/images/0561/6934/38768.png)
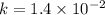
![\frac{1}{[C_4H_6]}](/tpl/images/0561/6934/fd07e.png) and the reaction is second order hence we get the rate law from
and the reaction is second order hence we get the rate law from ![k[A]^n](/tpl/images/0561/6934/3c428.png) .
. ![\frac{1}{[A]}=\frac{1}{[A]_0} +kt](/tpl/images/0561/6934/aad88.png) where A is
where A is 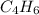 .
. 


