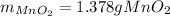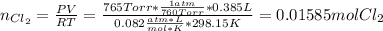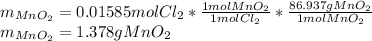Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid, HCl(aq) , as described by the chemical equation
MnO2(s)+4HCl(aq)⟶MnCl2(aq)+2H2O(l)+ Cl2(g)
How much MnO2(s) should be added to excess HCl(aq) to obtain 385 mL Cl2(g) at 25 °C and 765 Torr ? mass of MnO 2

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, jetblackcap
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 04:00, dustinsampsin2486
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions in other subjects:

Mathematics, 11.10.2019 11:50



Business, 11.10.2019 11:50




History, 11.10.2019 11:50


Chemistry, 11.10.2019 11:50