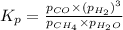Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 200.mL flask with 2.4 atm of methane gas and 3.9 atm of water vapor at 46.0°C. She then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 6.5 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, MJyoungboy
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3

Chemistry, 23.06.2019 00:30, natishtaylor1p8dirz
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 06:10, jamesgotqui6
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a. what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

Chemistry, 23.06.2019 07:30, sweetLips230
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1
You know the right answer?
Steam reforming of methane ( CH4 ) produces "synthesis gas," a mixture of carbon monoxide gas and hy...
Questions in other subjects:





History, 01.03.2020 01:56

Mathematics, 01.03.2020 01:56


Mathematics, 01.03.2020 01:56


Biology, 01.03.2020 01:57

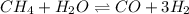
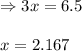
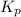 for above equation follows:
for above equation follows: