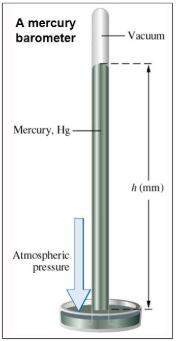
Chemistry, 19.03.2020 08:27 AllyJungkookie
A chemist makes a solution of Mg(NO3)2 by dissolving 21.3 g Mg(NO3)2 in water to make 100.0 mL of solution. What is the concentration of NO3− ions in the solution? Assume that Mg(NO3)2 is the only solute in the solution. The molar mass of Mg(NO3)2 is 148.33 g/mol.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2

Chemistry, 22.06.2019 21:30, liamgreene90
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3


You know the right answer?
A chemist makes a solution of Mg(NO3)2 by dissolving 21.3 g Mg(NO3)2 in water to make 100.0 mL of so...
Questions in other subjects:



Mathematics, 28.01.2020 22:45





Biology, 28.01.2020 22:45


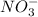 is, 2.88 M
is, 2.88 M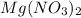 = 21.3 g
= 21.3 g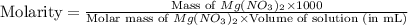
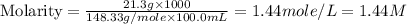
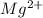 ion and 2 mole of
ion and 2 mole of