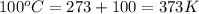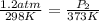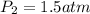Chemistry, 19.03.2020 06:38 ochoanene822
A sample of air occupies 3.8 L when the pressure is 1.2 atm and temperature at 25 Celsius. What pressure( in atm) will the gas have at 100 Celsius when the volum is held constant?

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, board1692
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 04:00, fantasticratz2
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

You know the right answer?
A sample of air occupies 3.8 L when the pressure is 1.2 atm and temperature at 25 Celsius. What pres...
Questions in other subjects:

Physics, 16.12.2021 19:40

Mathematics, 16.12.2021 19:40

Law, 16.12.2021 19:40

English, 16.12.2021 19:40

Mathematics, 16.12.2021 19:40





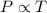
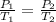
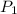 = initial pressure of gas = 1.2 atm
= initial pressure of gas = 1.2 atm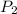 = final pressure of gas = ?
= final pressure of gas = ?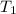 = initial temperature of gas =
= initial temperature of gas = 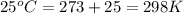
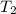 = final temperature of gas =
= final temperature of gas =