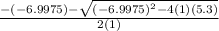Chemistry, 12.03.2020 21:40 daniellaZemira
Suppose a 250.0 mL flask is filled with 1.3 mol of I2 and 1.0 mol of HI. The following reaction becomes possible:
H2 (g) +I2 (g) ⇆ 2HI (g)
The equilibrium constant for this reaction is 0.983 at the temperature of the flask.
Calculate the equilibrium molarity of HI. Round your answer to two decimal places.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2



Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3
You know the right answer?
Suppose a 250.0 mL flask is filled with 1.3 mol of I2 and 1.0 mol of HI. The following reaction beco...
Questions in other subjects:


Business, 23.10.2019 20:50








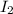 = 1.3 mol
= 1.3 mol = 1.0 mole
= 1.0 mole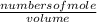
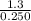
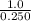
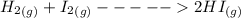
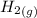 +
+ 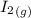 ----->
-----> 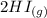
![K = \frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0545/4972/78f4e.png)
![K = \frac{[4-2x]^2}{[x][5.20+x]}](/tpl/images/0545/4972/b30d0.png) where K = 0.983
where K = 0.983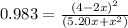
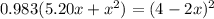
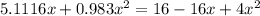
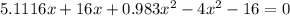
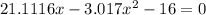
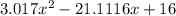
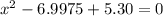
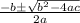
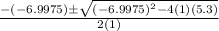
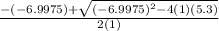 OR
OR