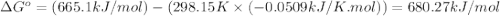Chemistry, 02.03.2020 21:22 jocelynmarquillo1
"Thermite" reactions have been used for welding metal parts such as railway rails and in metal refining. One such thermite reaction is 3 Mg(s) + Cr2O3(s) → 3 MgO(s) + 2 Cr(s). During the reaction, the surroundings absorb 665.1 kJ/mol of heat. Is the reaction spontaneous at room temperature (298.15 K) under standard conditions?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 20:30, dinapaul424
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1


Chemistry, 23.06.2019 02:10, sativataurus
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
You know the right answer?
"Thermite" reactions have been used for welding metal parts such as railway rails and in metal refin...
Questions in other subjects:










![\Delta S^o_{rxn}=\sum [n\times \Delta S^o_{(product)}]-\sum [n\times \Delta S^o_{(reactant)}]](/tpl/images/0530/7449/52737.png)
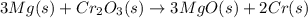
![\Delta S^o_{rxn}=[(3\times \Delta S^o_{(MgO(s))})+(2\times \Delta S^o_{(Cr(s))})]-[(3\times \Delta S^o_{(Mg(s))})+(1\times \Delta S^o_{(Cr_2O_3(s))})]](/tpl/images/0530/7449/80d1e.png)
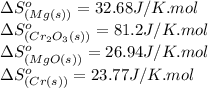
![\Delta S^o_{rxn}=[(3\times (26.94))+(2\times (23.77))]-[(3\times (32.68))+(1\times (81.2))]\\\\\Delta S^o_{rxn}=-50.88J/K=-0.0509kJ/K.mol](/tpl/images/0530/7449/fa231.png)
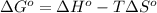
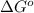 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?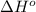 = standard enthalpy change of the reaction = 665.1 kJ/mol
= standard enthalpy change of the reaction = 665.1 kJ/mol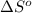 = standard entropy change of the reaction = -0.0509 kJ/K.mol
= standard entropy change of the reaction = -0.0509 kJ/K.mol