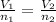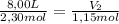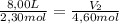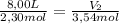Chemistry, 02.03.2020 19:49 barstr9146
A sample containing 2.30 mol of Ne gas has an initial volume of 8.00 L. What is the final volume, in liters, when the following changes occur in the quantity of the gas at constant pressure and temperature?a. A leak allows one-half of Ne atoms to escape. b. A sample of 3.50 mol of Ne is added to the 2.30 mol of Ne gas in the container. c. A sample of 25.0 g of Ne is added to the 2.30 mol of Ne gas in the container.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, 2019reynolds
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 12:30, masteroftheuniverse3
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
A sample containing 2.30 mol of Ne gas has an initial volume of 8.00 L. What is the final volume, in...
Questions in other subjects: