Consider the following elementary reaction:
N2O—> N2 + O
suppose we let K1 stand fo...

Consider the following elementary reaction:
N2O—> N2 + O
suppose we let K1 stand for the rate constant of this reaction, and k-1 stand for the rate constant of the reverse reaction. write an expression that gives the equilibrium concentration of N2O in terms of k1, k-1 and the equilibrium concentrations of N2 and O

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 13:30, suemmimonjaras8374
The atomic number, or number, is the described as the number of in the nucleus of an chemical element.
Answers: 1

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Questions in other subjects:


Biology, 13.02.2021 14:40

Computers and Technology, 13.02.2021 14:40

Mathematics, 13.02.2021 14:40


English, 13.02.2021 14:40

English, 13.02.2021 14:40



Mathematics, 13.02.2021 14:40

![[N_2O]=\frac{K^{-1}}{K_1}\times [N_2][O_2]](/tpl/images/0524/0986/7a4ba.png)
![R_f=K_1\times [N_2O]](/tpl/images/0524/0986/5d806.png)
![R_b=K^{-1}\times [N_2][O_2]](/tpl/images/0524/0986/327e3.png)
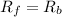
![K_1\times [N_2O]=K^{-1}\times [N_2][O_2]](/tpl/images/0524/0986/21d13.png)


