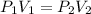Chemistry, 20.02.2020 22:05 luludawn3874
In response to Boyle’s Law, the pressure of a gas increases as the volume decreases because
the gas particles strike the walls of the container more often
the temperature of the gas increases
the gas particles get bigger
the gas particles strike the walls of the container with more force

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, valeriekbueno
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1
You know the right answer?
In response to Boyle’s Law, the pressure of a gas increases as the volume decreases because
Questions in other subjects:

Biology, 04.09.2020 15:01




English, 04.09.2020 16:01

Health, 04.09.2020 16:01

History, 04.09.2020 16:01

Biology, 04.09.2020 16:01

History, 04.09.2020 16:01