
Chemistry, 20.02.2020 08:37 PolinaZagorodniy123
Aluminum will react with bromine to form aluminum bromide (used as an acid catalyst in organic synthesis). Al(s) + Br2(l) → Al2Br6(s) [unbalanced] How many moles of Al are needed to form 2.43 mol of Al2Br6?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, carter1809
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Aluminum will react with bromine to form aluminum bromide (used as an acid catalyst in organic synth...
Questions in other subjects:


English, 21.07.2021 01:00

Mathematics, 21.07.2021 01:00

Mathematics, 21.07.2021 01:00

English, 21.07.2021 01:00


History, 21.07.2021 01:00

Mathematics, 21.07.2021 01:00


SAT, 21.07.2021 01:00

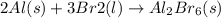
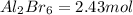
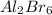 is obtained from 2 moles of aluminum.
is obtained from 2 moles of aluminum.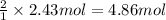 of Al
of Al

