
Chemistry, 21.07.2021 01:00 QueenZenobia
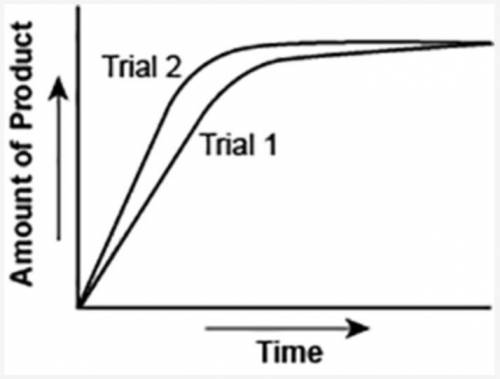
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A: Trial 1, because the average rate of the reaction is lower.
B: Trial 1, because this reaction lasted for a longer duration than Trial 2.
C: Trial 2, because this reaction was initially fast and later slowed down.
D: Trial 2, because the volume of product formed per unit time was higher.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1



Chemistry, 23.06.2019 03:30, uniqueray33
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions in other subjects:

History, 22.11.2021 18:30

Mathematics, 22.11.2021 18:30



Mathematics, 22.11.2021 18:40







