

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, astultz309459
What is the molecular formula of a hydrocarbon with m+ = 166? (write the formula with no subscripts, e. g. c4h10.) what is the sum of rings and double bonds in this compound?
Answers: 1

Chemistry, 21.06.2019 18:30, tiwaribianca475
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2


Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1
You know the right answer?
Consider the decomposition of red mercury(II) oxide under standard state conditions. )H0 T SFE ڮ( H...
Questions in other subjects:

Mathematics, 27.06.2019 01:30

Spanish, 27.06.2019 01:30

Mathematics, 27.06.2019 01:30





Mathematics, 27.06.2019 01:30


History, 27.06.2019 01:30

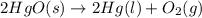
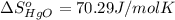
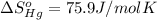
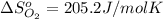
![\Delta S=[2\times \Delta S_{Hg}^o+1\times \Delta S_{O_2}^o]-[2\times \Delta S_{HgO}^o]](/tpl/images/0515/1320/fd534.png)
![=[2\times 75.9 J/mol K+1\times 205.2 J/molK]-[2\times 70.29 J/molK]=216.42 J/mol K](/tpl/images/0515/1320/fa874.png)


