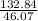Chemistry, 19.02.2020 01:04 hilario4785
Fermentation of 826 mL grape juice (density is 1.0 ) is allowed to take place in a bottle with a total volume of 885 mL until 19% by volume is ethanol (). Assuming that obeys Henry’s law, calculate the partial pressure of in the gas phase and the solubility of in the wine at 25°C. The Henry’s law constant for is mol/L ⋅ atm at 25°C with Henry’s law in the form , where is the concentration of the gas in mol/L. (The density of ethanol is 0.79 .)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1


Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
Fermentation of 826 mL grape juice (density is 1.0 ) is allowed to take place in a bottle with a tot...
Questions in other subjects:

Biology, 23.06.2020 22:01

Biology, 23.06.2020 22:01

Health, 23.06.2020 22:01

Mathematics, 23.06.2020 22:01






Mathematics, 23.06.2020 22:01