Chemistry, 12.02.2020 05:48 cristykianpour
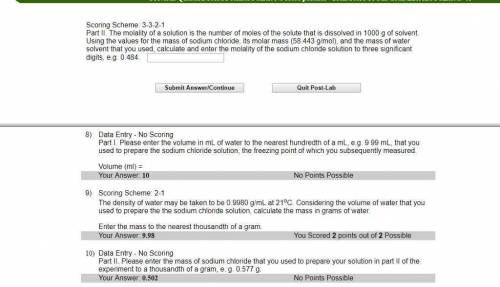
Part II. The molality of a solution is the number of moles of the solute that is dissolved in 1000 g of solvent. Using the values for the mass of sodium chloride, its molar mass (58.443 g/mol), and the mass of water solvent that you used, calculate and enter the molality of the sodium chloride solution to three significant digits, e. g. 0.484.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, bettybales1986
According to the tide table below what time of day will the highest tide occur?
Answers: 1



Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1
You know the right answer?
Part II. The molality of a solution is the number of moles of the solute that is dissolved in 1000 g...
Questions in other subjects:

Mathematics, 06.01.2021 02:20

English, 06.01.2021 02:20

Chemistry, 06.01.2021 02:20

English, 06.01.2021 02:20

Mathematics, 06.01.2021 02:20

Mathematics, 06.01.2021 02:20



English, 06.01.2021 02:20