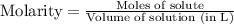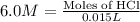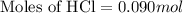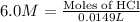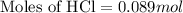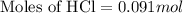Answers: 3


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 05:00, kayranicole1
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 08:00, daliakreynin
Which of the following notations would be the appropriate and final way to display the formula for magnesium chloride a. mgcl2 b. mg+2cl–1 c. mgcl2 d. mgcl
Answers: 2

Chemistry, 23.06.2019 10:30, taniyahbenyamin2
What’s the physical properties in calcium chloride
Answers: 1
You know the right answer?
Part iv. for each trial, calculate the number moles of 6.0 m hcl used in the reaction. report your a...
Questions in other subjects:



Mathematics, 15.08.2020 22:01