Consider a solution containing 0.181 m lead ions and 0.365
miron(ii) ions. the ksp for lead su...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, hoytkeke6776
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2
You know the right answer?
Questions in other subjects:


English, 04.04.2021 04:00

Mathematics, 04.04.2021 04:00



History, 04.04.2021 04:00

Mathematics, 04.04.2021 04:00

Mathematics, 04.04.2021 04:00


![[H_3O^+]=6.8*10^{-22}](/tpl/images/0448/0948/8039f.png)
![[Pb^{+2}]=1*10^{-6}](/tpl/images/0448/0948/21ac4.png) :
:![Kps=[Pb^{+2}]*[S^{-2}]=3.4*10^{-28}](/tpl/images/0448/0948/0133d.png)
![1*10^{-6}*[S^{-2}]=3.4*10^{-28}](/tpl/images/0448/0948/5bca8.png)
![[S^{-2}]=3.4*10^{-22}](/tpl/images/0448/0948/c45b5.png)
![Kps=[0.365]*[S^{-2}]=3.7*10^{-19}](/tpl/images/0448/0948/c04d1.png)
![[S^{-2}]=1.01*10^{-18}](/tpl/images/0448/0948/5c3d9.png)
![[S^{-2}]](/tpl/images/0448/0948/d1351.png) concentration required to start precipitating iron is higher than the final concentration for the Pb precipitation (when the desired concentration is reached), the iron will not precipitate.
concentration required to start precipitating iron is higher than the final concentration for the Pb precipitation (when the desired concentration is reached), the iron will not precipitate. 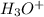
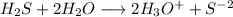
![[H_3O^+]=2*[S^{-2}]=6.8*10^{-22}](/tpl/images/0448/0948/3de14.png)


