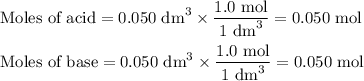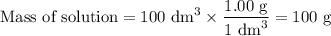Chemistry, 05.01.2020 17:31 gharrell03
50cm3 of 1 mol/dm3 hcl at 30°c was mixed with 50cm3 of 1mol/dm3 naoh at 30°c in a styrofoam calorimeter. the temperature of the calorimeter rose by 4.5°c. calculate the heat of reaction per mol of h20 formed.( heat capacity of the calorimeter is 50j/°c

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, jessicasbss6840
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 22:00, notearslefttocry14
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
50cm3 of 1 mol/dm3 hcl at 30°c was mixed with 50cm3 of 1mol/dm3 naoh at 30°c in a styrofoam calorime...
Questions in other subjects:

History, 11.10.2019 18:10

Mathematics, 11.10.2019 18:10

Mathematics, 11.10.2019 18:10



Geography, 11.10.2019 18:10