Consider the following standard heats of formation:
p₄o₁₀(s) = -3110 kj/mol
h₂o(l) =...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, dpazmembreno
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1


You know the right answer?
Questions in other subjects:

Mathematics, 29.01.2021 16:30










![\Delta H_{f}^{\circ } [P_{4}O_{10}(s)]](/tpl/images/0435/2502/5b047.png) = -3110 kJ/mol,
= -3110 kJ/mol,![\Delta H_{f}^{\circ } [H_{2}O(l)]](/tpl/images/0435/2502/46f61.png) = -286 kJ/mol,
= -286 kJ/mol, ![\Delta H_{f}^{\circ } [H_{3}PO_{4}(s)]](/tpl/images/0435/2502/1d826.png) = -1279 kJ/mol
= -1279 kJ/mol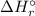 = ?
= ?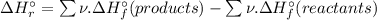
![\Delta H_{r}^{\circ } = [4 \times \Delta H_{f}^{\circ } [H_{3}PO_{4}(s)]] - [1 \times \Delta H_{f}^{\circ } [P_{4}O_{10}(s)] + 6 \times \Delta H_{f}^{\circ } [H_{2}O(l)]]](/tpl/images/0435/2502/c9ebf.png)
![\Rightarrow \Delta H_{r}^{\circ } = [4 \times (-1279\, kJ/mol)] - [1 \times (-3110\, kJ/mol) + 6 \times (-286\, kJ/mol)]](/tpl/images/0435/2502/ea7a2.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-5116\, kJ] - [-3110\, kJ -1716\, kJ]](/tpl/images/0435/2502/7e6ce.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-5116\, kJ] - [-4826\, kJ] = -290\,kJ](/tpl/images/0435/2502/ee612.png)


