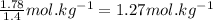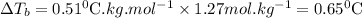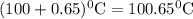Chemistry, 24.12.2019 20:31 tracyaleblanc
What is the boiling point of a solution produced by adding 610 g of cane sugar (molar mass 342.3 g/mol) to 1.4 kg of water? for each mole of nonvolatile solute, the boiling point of 1 kg of water is raised 0.51 ∘c.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 15:50, Edwardwall
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
What is the boiling point of a solution produced by adding 610 g of cane sugar (molar mass 342.3 g/m...
Questions in other subjects:


Mathematics, 28.09.2019 07:30

Mathematics, 28.09.2019 07:30

Mathematics, 28.09.2019 07:30

Social Studies, 28.09.2019 07:30




Physics, 28.09.2019 07:30

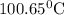
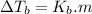
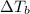 is elivation in boiling point of solution,
is elivation in boiling point of solution, 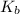 is ebbulioscopic constant of solvent (how much temperature is raised for dissolution of 1 mol of non-volatile solute) and m is molality of solution.
is ebbulioscopic constant of solvent (how much temperature is raised for dissolution of 1 mol of non-volatile solute) and m is molality of solution.
 moles of cane sugar
moles of cane sugar