Chemistry, 19.12.2019 22:31 mostman077
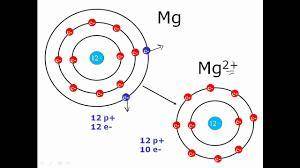
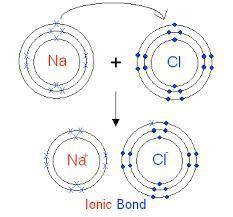
Elaborate on how the duet and octet rules relate to ion formation?
a) the duet rule or the octet rule state that atoms form ions in an effort to acquire valence electron configuration impermanence.
b) both the duet and octet rules reveal that atoms gain or lose electrons forming ions to achieve a more stable electron configuration.
c) ion formation only occur if the atom have a full duet electron configuration or full octet electron configuration of valence electrons.
d) formation of immutable noble-gas configuration ions results from atoms with ambiguous duet or octet configurations of valence electrons.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, milkshakegrande101
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Elaborate on how the duet and octet rules relate to ion formation?
a) the duet rule or the o...
a) the duet rule or the o...
Questions in other subjects:








Mathematics, 30.03.2020 21:16


Social Studies, 30.03.2020 21:16