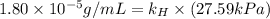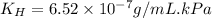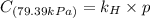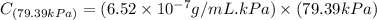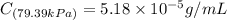Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, elizabethprasad2
How many grams of n2h4 will be consumed by 23 g of n2o4
Answers: 1



Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Exposing a 250 ml sample of water at 20.∘c to an atmosphere containing a gaseous solute at 27.59 kpa...
Questions in other subjects:

Physics, 27.09.2021 14:00


History, 27.09.2021 14:00




English, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00

History, 27.09.2021 14:00

Mathematics, 27.09.2021 14:00

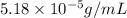
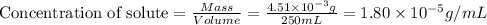
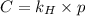
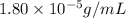
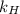 = Henry's law constant = ?
= Henry's law constant = ?