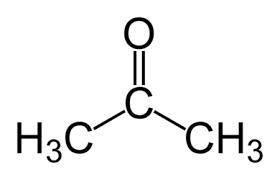
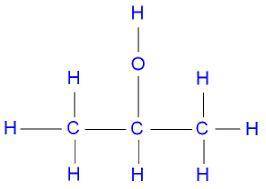
Identify the oxidation number of the highlighted carbon atoms in each of the molecules.
...

Chemistry, 14.12.2019 06:31 2023jpeterson
Identify the oxidation number of the highlighted carbon atoms in each of the molecules.
a. in molecule a a central carbon atom is bonded to two c h 3 groups and an o atom through a double bond.
b. the central carbon atom is highlighted.
c. in molecule b, a central carbon atom is bonded to two c h 3 groups, an o h group, and an h atom.
d. the central carbon atom is highlighted.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 17:30, TheViperMlg23676
What causes most sediment to wash or fall into a river
Answers: 1

Chemistry, 22.06.2019 19:00, hmontalvo22
How many moles are contained in 5.6 l of h2 at stp
Answers: 3
You know the right answer?
Questions in other subjects:


Mathematics, 06.11.2019 00:31


Mathematics, 06.11.2019 00:31




Mathematics, 06.11.2019 00:31

Biology, 06.11.2019 00:31

Mathematics, 06.11.2019 00:31

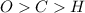
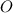 always gets -1 and
always gets -1 and 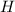 always gets +1 in these two compounds.The carbon - carbon bond will give 0 oxidation state.
always gets +1 in these two compounds.The carbon - carbon bond will give 0 oxidation state.