
For the following reaction, 53.7 grams of iron(iii) oxide are allowed to react with 22.8 grams of aluminum. iron(iii) oxide (s) + aluminum (s) aluminum oxide (s) + iron (s) what is the maximum amount of aluminum oxide that can be formed? grams what is the formula for the limiting reagent? what amount of the excess reagent remains after the reaction is complete? grams

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Playboycxm
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 17:20, holmesleauja
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 19:00, HaydenSturgis1
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
For the following reaction, 53.7 grams of iron(iii) oxide are allowed to react with 22.8 grams of al...
Questions in other subjects:

Mathematics, 11.07.2019 12:30

Advanced Placement (AP), 11.07.2019 12:30




English, 11.07.2019 12:30

History, 11.07.2019 12:30

Mathematics, 11.07.2019 12:30

Geography, 11.07.2019 12:30

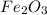 .
.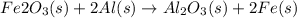
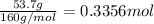
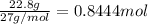
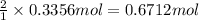 of aluminum.
of aluminum.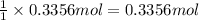 of aluminum oxide
of aluminum oxide

