Chemistry, 10.12.2019 03:31 hunterbrod9903
Calculate the reaction quotient qp for the following redox reaction: 14h+ + cr2o72- + 6cl- > 2cr3+ + 3cl2 + 7h2o the reaction mixture has ph = 0.0, [cr2o72-] = 1.0 m, [cl-] = 1.0 m, [cr3+] = 0.10 m, and parital pressure of chlorine gas of 0.010 atm.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1


Chemistry, 22.06.2019 12:00, BakerElsie02
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Calculate the reaction quotient qp for the following redox reaction: 14h+ + cr2o72- + 6cl- > 2c...
Questions in other subjects:

English, 20.09.2020 16:01






English, 20.09.2020 16:01


Mathematics, 20.09.2020 16:01

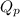 for the given redox reaction is
for the given redox reaction is 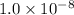
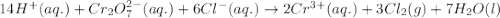
![Q_{p}=\frac{[Cr^{3+}]^{2}.P_{Cl_{2}}^{3}}{[H^{+}]^{14}.[Cr_{2}O_{7}^{2-}].[Cl^{-}]^{6}}](/tpl/images/0411/1433/37c18.png)
 is taken as 1 due to the fact that
is taken as 1 due to the fact that ![pH=-log[H^{+}]](/tpl/images/0411/1433/863f0.png)
![[H^{+}]=10^{-pH}](/tpl/images/0411/1433/6c9d3.png)