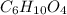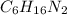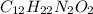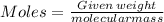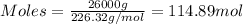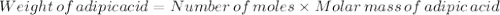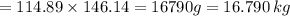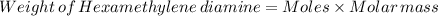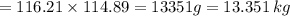Chemistry, 09.12.2019 21:31 lilday8230
Nylon 6,6 may be formed by means of a condensation polymerization reaction in which hexamethylene diamine [nh2-(ch2)6-nh2] and adipic acid react with one another with the formation of water as a byproduct. what masses of (a) hexamethylene diamine and (b) adipic acid are necessary to yield 26 kg of completely linear nylon 6,6? this polymerization reaction occurs according to the following equation:

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

You know the right answer?
Nylon 6,6 may be formed by means of a condensation polymerization reaction in which hexamethylene di...
Questions in other subjects:

Physics, 27.04.2021 03:40


Mathematics, 27.04.2021 03:40


Mathematics, 27.04.2021 03:40

Mathematics, 27.04.2021 03:40


Mathematics, 27.04.2021 03:40