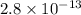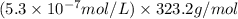Chemistry, 05.12.2019 18:31 BrainlyAvenger
G[mcquarrie 22-7] the value of ksp for pbcro4(s) in equilibrium with water at 25◦c is 2.8 · 10−13m2 . write the chemical equation that represents the solubility equilibrium for pbcro4(s) and calculate its solubility in grams per liter in water at 25◦c.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1

Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 04:31, cassiuspricerules
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
G[mcquarrie 22-7] the value of ksp for pbcro4(s) in equilibrium with water at 25◦c is 2.8 · 10−13m2...
Questions in other subjects:

Mathematics, 28.07.2019 12:00


Mathematics, 28.07.2019 12:00


Mathematics, 28.07.2019 12:00



Advanced Placement (AP), 28.07.2019 12:00


Mathematics, 28.07.2019 12:00


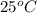 is,
is, 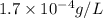
![K_{sp}=[Pb^{2+}][CrO_4^{2-}]](/tpl/images/0404/8064/04f28.png)


 =
=