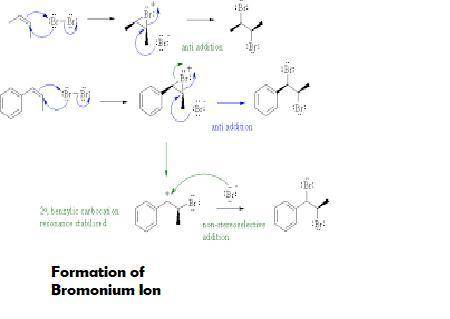
Two different bromonium ions are formed because br2 can add to the double bond either from the top of the plane or from the bottomtwo different bromonium ions are formed because {\rm br_2} can add to the double bond blank of the plane defined by the double bond, and the two bromonium ions are formed in equal amounts. of the plane defined by the double bond, and the two bromonium ions are formed in equal amounts.
a) true
b) false

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, johngayden46
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2



Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
Two different bromonium ions are formed because br2 can add to the double bond either from the top o...
Questions in other subjects:


Mathematics, 14.11.2019 17:31







Biology, 14.11.2019 17:31