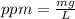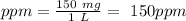Chemistry, 28.11.2019 05:31 jellyangie1
Astock solution contains a mixture of ~100 ppm chloride, fluoride, nitrite, bromide, nitrate and phosphate anions. in order to prepare 1 l of 100 ppm nitrite stock solution, you weigh out 150.0 mg of nano2. the actual concentration of nitrite would be:

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 01:00, kaykardash
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2
You know the right answer?
Astock solution contains a mixture of ~100 ppm chloride, fluoride, nitrite, bromide, nitrate and pho...
Questions in other subjects:


Biology, 22.11.2020 14:10




Biology, 22.11.2020 14:10


Social Studies, 22.11.2020 14:10


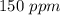
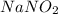 , so:
, so: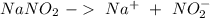
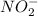 is 1:1
is 1:1