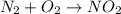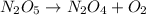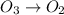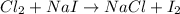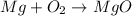Be sure to answer all parts. write an unbalanced equation to represent each of the following reactions: do not include phase abbreviations. (a) nitrogen and oxygen react to form nitrogen dioxide. (b) dinitrogen pentoxide reacts to form dinitrogen tetroxide and oxygen. (c) ozone reacts to form oxygen. (d) chlorine and sodium iodide react to form iodine and sodium chloride. (e) magnesium and oxygen react to form magnesium oxide.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 03:00, EllaLovesAnime
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
Be sure to answer all parts. write an unbalanced equation to represent each of the following reactio...
Questions in other subjects:

History, 06.07.2019 18:30


English, 06.07.2019 18:30

History, 06.07.2019 18:30