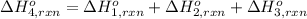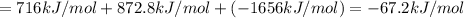Chemistry, 22.11.2019 03:31 CyberSongWriter
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estimate the standard enthalpy of formation of methane (ch4). c(s) → c(g) δh o rxn = 716 kj/mol 2h2(g) → 4h(g) δh o rxn = 872.8 kj/mol

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 23:40, sydneykated
The kw for water at 0 °c is 0.12× 10–14 m2. calculate the ph of a neutral aqueous solution at 0 °c.
Answers: 2
You know the right answer?
Using the following information and the fact that the average c―h bond enthalpy is 414 kj/mol, estim...
Questions in other subjects:

English, 25.08.2019 17:20


Social Studies, 25.08.2019 17:20

History, 25.08.2019 17:20


Geography, 25.08.2019 17:20

Mathematics, 25.08.2019 17:20



Health, 25.08.2019 17:20

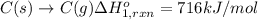 ...[1]
...[1]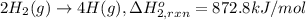 ...[2]
...[2]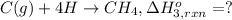 ...[3]
...[3]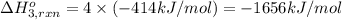
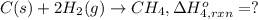 ...[4]
...[4]