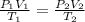Chemistry, 25.08.2019 17:20 novesparks
1. when the kelvin temperature of an enclosed gas doubles, the particles of the gas (1 point)
move faster
strike the walls of the container with less force
decrease in average kinetic energy
decrease in volume
2. the volume of a gas is reduced from 4 l to 0.5 l while the temperature is held constant. how (1 point)
2. the volume of a gas is reduced from 4 l to 0.5 l while the temperature is held constant. how
does the gas pressure change?
(1 point)
it increases by a factor of four.
it decreases by a factor of eight.
it increases by a factor of eight.
it increases by a factor of two.
3. charles's law states that (1 point)
the pressure of a gas is inversely proportional to its temperature in kelvins
the volume of a gas is directly proportional to its temperature in kelvins
the pressure of a gas is directly proportional to its temperature in kelvins
the volume of a gas is inversely proportional to its temperature in kelvins
4. a sample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c? (1 point)
10.6 ml
27 ml
36 ml
8.0 ml
5. the combined gas law relates which of the following? (1 point)
pressure and volume only
temperature and pressure only
volume and temperature only
temperature, pressure, and volume

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:10, lilque6112
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:00, nana54muller
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

You know the right answer?
1. when the kelvin temperature of an enclosed gas doubles, the particles of the gas (1 point)
...
...
Questions in other subjects:










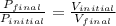
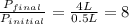
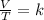
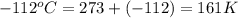
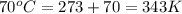
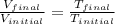
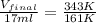
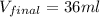
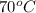 is, 36 ml
is, 36 ml