
Binary compounds of alkali metals and hydrogen react with water to liberate hydrogen gas. the hydrogen gas from the reaction of a sample of sodium hydride with an excess of water fills a volume of 0.475 l above the water. the temperature of the gas is 35 ∘c and the total pressure is 755 mmhg.
find the mass of h2 liberated and the mass of nah that reacted.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, maryjane8872
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
Binary compounds of alkali metals and hydrogen react with water to liberate hydrogen gas. the hydrog...
Questions in other subjects:





Spanish, 06.10.2021 17:40


Arts, 06.10.2021 17:40


Mathematics, 06.10.2021 17:40

Mathematics, 06.10.2021 17:40

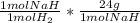 = 0.4480 g NaH
= 0.4480 g NaH

