Chemistry, 16.11.2019 02:31 adlytle6506
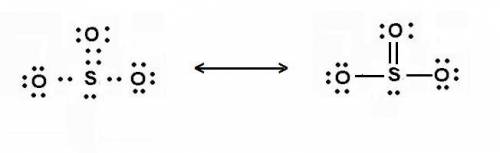
Draw the lewis structure for so32−, including lone pairs. select draw rings more erase select draw rings more erase select draw rings more erase select draw rings more erase s o what is the molecular shape of so32−? bent trigonal pyramidal linear trigonal planar tetrahedral what is the o−s−o bond angle? 180∘ < 109.5∘ 109.5∘ 120∘ the s−o bond in so32− is the molecule so32− is

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 07:30, genyjoannerubiera
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
You know the right answer?
Draw the lewis structure for so32−, including lone pairs. select draw rings more erase select draw r...
Questions in other subjects:




Health, 01.06.2020 08:58

Chemistry, 01.06.2020 08:58


Geography, 01.06.2020 08:58


Mathematics, 01.06.2020 08:58

Mathematics, 01.06.2020 08:58

 is trigonal pyramidal and bond angle between O-S-O is
is trigonal pyramidal and bond angle between O-S-O is 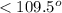
![\text{Number of electron pair}=\frac{1}{2}[V+N-C+A]](/tpl/images/0376/9107/9e987.png)
![\text{Number of electrons}=\frac{1}{2}\times [6+2]=4](/tpl/images/0376/9107/2c46b.png)
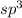 and the electronic geometry of the molecule will be tetrahedral.
and the electronic geometry of the molecule will be tetrahedral. due to repulsion between lone and bond pair of electrons.
due to repulsion between lone and bond pair of electrons.