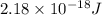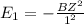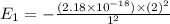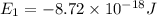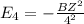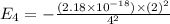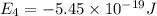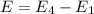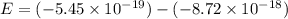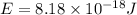Chemistry, 13.11.2019 04:31 kawaunmartinjr10
Simplified. the absorption spectra of ions have been used to identify the presence of the elements in the atmospheres of the sun and other stars. what is the energy of a photon (in j) that is absorbed by he+ ions, when an electron is excited from the bohr orbit with n = 1 to the n = 4 state? the energy of an electron in the nth level is

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, kaitlyn2030
How do the energy differences between the higher energy levels of an atom compare with the energy difference between the lower energy level of the atom
Answers: 1

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 03:30, acaciacoats
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2
You know the right answer?
Simplified. the absorption spectra of ions have been used to identify the presence of the elements i...
Questions in other subjects:

Physics, 06.09.2019 22:30









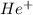 ions is
ions is 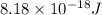
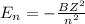
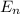 = energy of an electron in the nth level
= energy of an electron in the nth level