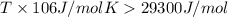Chemistry, 06.09.2019 22:30 muanghoih14
For a reaction for which ah = +29.3 kj/mol and as = +106 j/mol•k, which of the following statements is true? a) the reaction is spontaneous above 276 k. b) the reaction is spontaneous below 276 k. c) the reaction will never reach equilibrium. d) the reaction will never be spontaneous.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
For a reaction for which ah = +29.3 kj/mol and as = +106 j/mol•k, which of the following statements...
Questions in other subjects:


Mathematics, 05.07.2019 06:50

English, 05.07.2019 06:50



Health, 05.07.2019 06:50


Mathematics, 05.07.2019 06:50


Social Studies, 05.07.2019 06:50

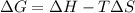
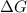 = Gibbs free energy
= Gibbs free energy 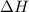 = enthalpy change = +29.3 kJ/mol =29300 J/mol
= enthalpy change = +29.3 kJ/mol =29300 J/mol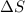 = entropy change = +106 J/molK
= entropy change = +106 J/molK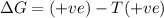
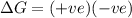
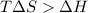 for reaction to be spontaneous
for reaction to be spontaneous