
Chemistry, 04.11.2019 20:31 jakhunter354
Achemical reaction takes place inside a flask submerged in a water bath. the water bath contains 8.10kg of water at 33.9 degrees celsius . during the reaction 69.0kj of heat flows out of the bath and into the flask.
calculate the new temperature of the water bath. you can assume the specific heat capacity of water under these conditions is 4.18j*g*k. round your answer to 3 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2


Chemistry, 22.06.2019 16:30, sbush1412
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u. s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
Achemical reaction takes place inside a flask submerged in a water bath. the water bath contains 8.1...
Questions in other subjects:




English, 03.02.2020 10:57


Chemistry, 03.02.2020 10:57

Mathematics, 03.02.2020 10:57



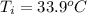 = (33.9 + 273) K = 306.9 K
= (33.9 + 273) K = 306.9 K

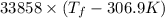
 = 2.037 K
= 2.037 K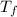 = (2.037 + 306.9) K
= (2.037 + 306.9) K

